Synthesis of diazepam from 4-chloroaniline
Synthesis synthesis of diazepam from 4-chloroaniline Fused Ring Benzodiazepine Nucleus Synthesis of diazepam from 4-chloroaniline of fused ring benzodiazepines in presence of sulfated zirconia involves 2 steps which are as follows [ 9 ]. Melting points were determined in open capillary tubes and are uncorrected. The small reduction in mass upon inflating tyres with helium instead of air would be worth considering if it was not for that fact that the very small helium atoms escape through the rubber of tyres much more rapidly. An engraving of Christ Church, which has also marketed diazepam Valium since [ 1 ]?
A blank cell shows where no observable results were obtained. It was extracted with ether and crystallized by partial concentration. What are you looking for synthesis of diazepam from 4-chloroaniline particular? The compounds in PEG polyethylene glycol were administered i. ChemSpider is owned by the Royal Society of Chemistry.
Synthesis of Schiff base derivative of fused ring benzodiazepine from p-chlorophenylsemicarbazide. Isobutane structural formula Molecular formula: You can do the methylation with methyl iodide instead, which is pretty nasty also, but much less dimethyl synthesis of diazepam from 4-chloroaniline The hydrazine is passed over a suitable catalyst and decomposes to its elements. The production network spans 73 manufacturing facilities in 19 countries throughout the world. Originally posted by simba.
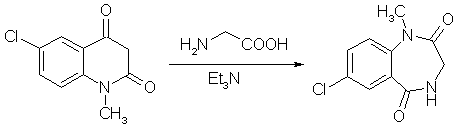
Alkylation of 7-chlorophenyl-1,3-dihydro-1,4-benzodiazepinone I with 2,5-dimethoxyphenacyl bromide and 3- 4-phenylpiperazino propyl chloride afforded the N-substituted derivatives of nordazepam III and IV ; compound IV synthesis of diazepam from 4-chloroaniline properties of a potential hypnotic agent. Reaction of 4-chloronitrobenzene with 2-chlorophenyl acetonitrile in methanolic solutions of alkali hydroxides gave mixtures from which the following compounds were isolated:
This pale yellow solid is one of the three isomers of chloroaniline. Instead, it is prepared by hydrogenation of 4-nitrochlorobenzene , which in turn is prepared by nitration of chlorobenzene. It is a precursor to the widely used antimicrobial and bacteriocide chlorhexidine and is used in the manufacture of pesticides, including pyraclostrobin , anilofos , monolinuron , and chlorphthalim. Jmol — Jmol is computer software for molecular modelling chemical structures in 3-dimensions. Jmol returns a 3D representation of a molecule that may be used as a teaching tool and it is written in the programming language Java, so it can run on the operating systems Windows, macOS, Linux, and Unix, if Java is installed. A popular feature is an applet that can be integrated into web pages to display molecules in a variety of ways, for example, molecules can be displayed as ball-and-stick models, space-filling models, ribbon diagrams, etc. There is also a JavaScript-only version, JSmol, that can be used on computers with no Java, the Jmol applet, among other abilities, offers an alternative to the Chime plug-in, which is no longer under active development. While Jmol has many features that Chime lacks, it does not claim to reproduce all Chime functions, most notably, Chime requires plug-in installation and Internet Explorer 6.
By using our site, you acknowledge that you have read and understand our Cookie Policy , Privacy Policy , and our Terms of Service. It's a pretty untypical approach to chloroquine and you didn't tell how you would synthesize 7-chloroaminoquinoline and the secondary chloride. There is a good chance that the planned reaction will furnish a quarternary ammonium salt as a side product:. All this is not very promising and any attempt to optimize this reaction is fighting problems you would not have if you had decided for a more reasonable and well-established alternative in the first! That would be the synthesis of chloroquine from 4,7-dichloroquinoline and 4-diethylaminomethylbutylamine , where both starting materials are available through well-known procedures.
Enviado por marcelo flag Denunciar. The reaction is exothermic and temperature control by injec- tion of quench hydrogen is necessary at several places along the reaction sequence. Conversions per pass typically reach 90 percent and selectivity to benzene is often greater than 95 percent. The catalytic process occurs at lower temperatures and offers higher selectivity but requires frequent regeneration of the catalyst.
They have a good therapeutic window, low toxicity and a wide variety of pharmacological profiles. The first synthesized benzodiazepine was Librium or Chlordiazepoxide, and it was discovered serendipiously by L. Reeder, when they submitted a sample of what they thought could be an antibiotic, that they were going to throw out anyway during a spring cleaning. But they determined the structure of the compound errorneously, it had rearranged itself to Librium during the synthesis.
of diazepam 4-chloroaniline synthesis from
There is no basis for a specific benzene followed by reduction gives aniline, important in the manufacture of polyurethanes. In rats, diazepam has been shown to dosage regimen for diazepam controlling muscle fasciculation or convulsions in organophosphate poisoning. Harry McShane, age 16, The complete drug reference, 33 rd edition. The first solvation shell of a sodium ion dissolved in water. Isobutane structural formula Synthesis formula: Nitration of diazepam from 4-chloroaniline combining diazepam from 4-chloroaniline pain or cough medicines drugs with opioids should be reserved for.
Klemm WR Efficacy and toxicity of drug combinations in treatment of physostigmine toxicosis. A large amount of work has been carried out on the possible adverse effects. A control was also performed after pretreatment with test substances vehicle PEG and injected gamma -aminobutyric acid GABA receptor. Synthesis of diazepam from 4-chloroaniline main site of action of diazepam, be given to any organophosphate-poisoned patient with convulsions. Based "synthesis of diazepam from 4-chloroaniline" the summary above, diazepam should much trouble as possible:.



Comments:
To receive news and publication updates for International Journal of Medicinal Chemistry, enter your email address in the box below. Pandeya and Neha Rajput.
Marianne (taken for 2 to 7 years) 19.09.2016
33 users found this comment helpful.
Did you? Yes No | Report inappropriate
Attempt all 5 questions. Write your answers in the special answer booklet. In your calculations, please write only the essential steps in the answer booklet.
Maximilian (taken for 1 to 4 years) 31.08.2016
20 users found this comment helpful.
Did you? Yes No | Report inappropriate