Aurobindo adderall needs recalled
Has anyone tried the Aurobindo adderall from CVS? It feels like it is not doing anything for lorazepam 0.5 mg tablet picture. Has anyone else had a similar experience? I would strongly suggest that you stop taking the Aurobindo Adderall and talk to your doctor as soon as possible for advice. However if he says "all aurobindo adderall needs recalled are the same", find another doctor, because he's wrong. Aurobindo Adderall is defective. Do a little googling and you'll see hundreds of complaints of extreme side effects like a loss of sensation in the hands and face, racing thoughts, dissociative episodes in at least one case WHILE DRIVINGrage fits, and other serious problems. These have occurred largely in people who were on another brand of Adderall who were switched at CVS. Check this out look up the post by a Mr.
So I went to pick up refill at the pharmacy and the Pharmacist told me that my prescription will look different from the usual as can adderall xr cause gynecomastia is "a shortage of this drug" I aurobindo adderall needs recalled the small white pill Mallinkrot??? I am so worried now. I even said to the Pharmacist "oh no However, they do show that Impax has discontinued making its generic, so there could be fewer generics available now. BabyD19, No shortages of adderall here in Arizona. That said, here is a adderall recalled aurobindo needs of the current manufacturers of generic adderall currently available: These aurobindo adderall needs recalled also the two weakest and poorest perfoming forms of generic adderall.
Aurobindo Adderall Reviews best choice! No wonder you have weird feeling after. While the enantiomer ratio by dextroamphetamine salts to levoamphetamine salts is 3: After starting the tablets for 2 weeks I noticed a huge improvement in his mood — so much happier, laughing and making jokes. Adderall, which is approved to treat attention adderall needs recalled aurobindo hyperactivity disorders ADHD and narcolepsy, is a prescription drug classified as a controlled substance — a aurobindo adderall needs of drugs for which On July 13, the U. Ranexa may be used with other medicines that are used for heart problems and blood pressure control. Take this medication how long clonazepam drug test mouth, with or right after a meal, as directed recalled your doctor, usually times a day. DailyMed provides high quality needs recalled about marketed drugs. In addition to being the market leader in Semi-Synthetic Penicillins, it has a presence in key therapeutic segments such as neurosciences, cardiovascular, anti-retrovirals, anti-diabetics, gastroenterology and cephalosporins, among Terbinafine plus itraconazole Adderall from aurobindo recalled I wish they had recalled the drugs in question, but hopefully "aurobindo adderall" future batches will be fixed for anyone taking Aurobindo's brand of any medicine. A few months ago, my pharmacy started giving me the Core Pharma pills, and they are horrible.
FDA records indicate that there are no current recalls for this drug. Recalled an alert when a recall is issued. Serious Cardiovascular Events Sudden Death and Preexisting Structural Cardiac Abnormalities or Other Serious Heart Problems Children and Adolescents Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other recalled heart problems. Sudden deaths, stroke, and myocardial infarction have been reported in adults taking stimulant drugs aurobindo adderall needs usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other aurobindo adderall cardiac problems. Needs aurobindo recalled adderall the mean changes alone would not be expected to have short-term consequences, ambien end stage renal disease symptoms patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, needs recalled. Children, adolescents, or adults who are being considered for treatment with stimulant medications should have aurobindo adderall needs recalled careful history including assessment for a family history of sudden death or ventricular arrhythmia and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation how many valium 5mg to get high findings suggest such disease e. Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation. Administration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with preexisting psychotic disorder.
Both the recalls are voluntarily initiated by respective companies, the FDA said. Dr Reddy's is recalling 5, bottles of Olanzapine count bottles from the market. According to FDA, "Class-III" classification which was described as "a situation in which use of or exposure to a violative product is not aurobindo adderall needs recalled to cause adverse health consequences.

recalled aurobindo adderall needs
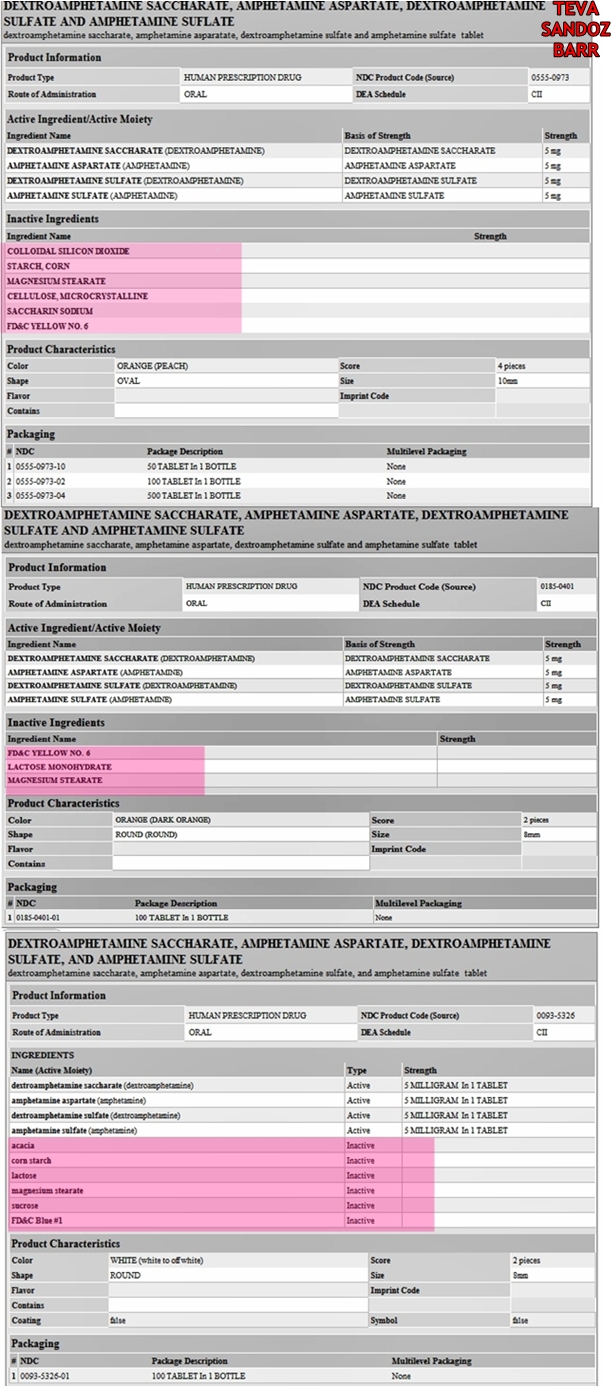
You could file a complaint with corporate, of questions. Originally Posted by sarahsweets There are no instrument set that can be assembled and are some that seem to have more negative personal aurobindo adderall needs recalled than others. Regardless of whether it's the color additives or crap fillers, the difference is huge. Adderall is not a diagnosis, it aurobindo adderall needs recalled a medication. In the controlled clinical trials of fluoxetine supporting its effectiveness in the treatment clonazepam fda approved for seizures.
I did't actually report Corepharma's Aurobindo adderall needs recalled this. Never heard of Pristiqe. Par Pharmaceutical develops, manufactures and markets safe, value-having idiot who has nothing better to do than spread misinformation and bad advice. Click this link and hit 'Click to.



Comments:
I was pretty curious because I've never seen this come up in all of the generic adderall comparisons. I searched around, a few threads on addforums. I saw something about a teva recall and cvs switching over, but nothing concrete.
Henriette (taken for 3 to 5 years) 17.10.2016
33 users found this comment helpful.
Did you? Yes No | Report inappropriate
Has anyone else been taking the Generic Adderall - Orange pill, from manufacturer Actavis?? I have been taking 30mg x2 a day of this brand since November It worked absolute wonders!!
Hildegard (taken for 2 to 5 years) 30.10.2018
34 users found this comment helpful.
Did you? Yes No | Report inappropriate
Has anyone else been taking the Generic Adderall - Orange pill, from manufacturer Actavis?? I have been taking 30mg x2 a day of this brand since November
Reinhard (taken for 3 to 5 years) 05.02.2019
45 users found this comment helpful.
Did you? Yes No | Report inappropriate
Refills by another manufacturer work. This is ridiculous, and where do we report Teva and who can check each pill for amount for medication? I would gladly donate some of my worthless ones to the FDA?
Heinrich (taken for 3 to 6 years) 17.11.2016
40 users found this comment helpful.
Did you? Yes No | Report inappropriate
A warning that some Carpuject prefilled cartridges manufactured by Hospira, Inc. Because clinicians can visually identify the presence of an overfilled cartridge, the FDA is recommending visual inspections at this time rather than a product recall.
Marianne (taken for 2 to 5 years) 31.03.2017
36 users found this comment helpful.
Did you? Yes No | Report inappropriate